CoolSculpting® Uses
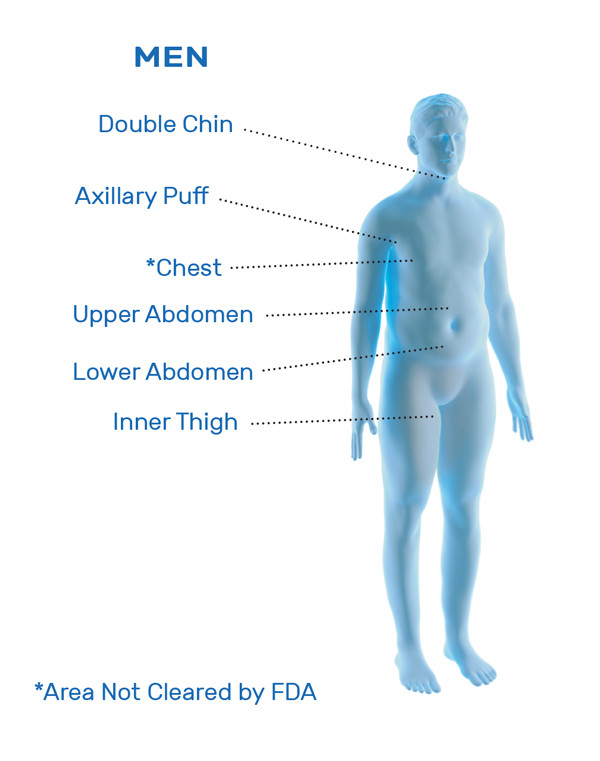
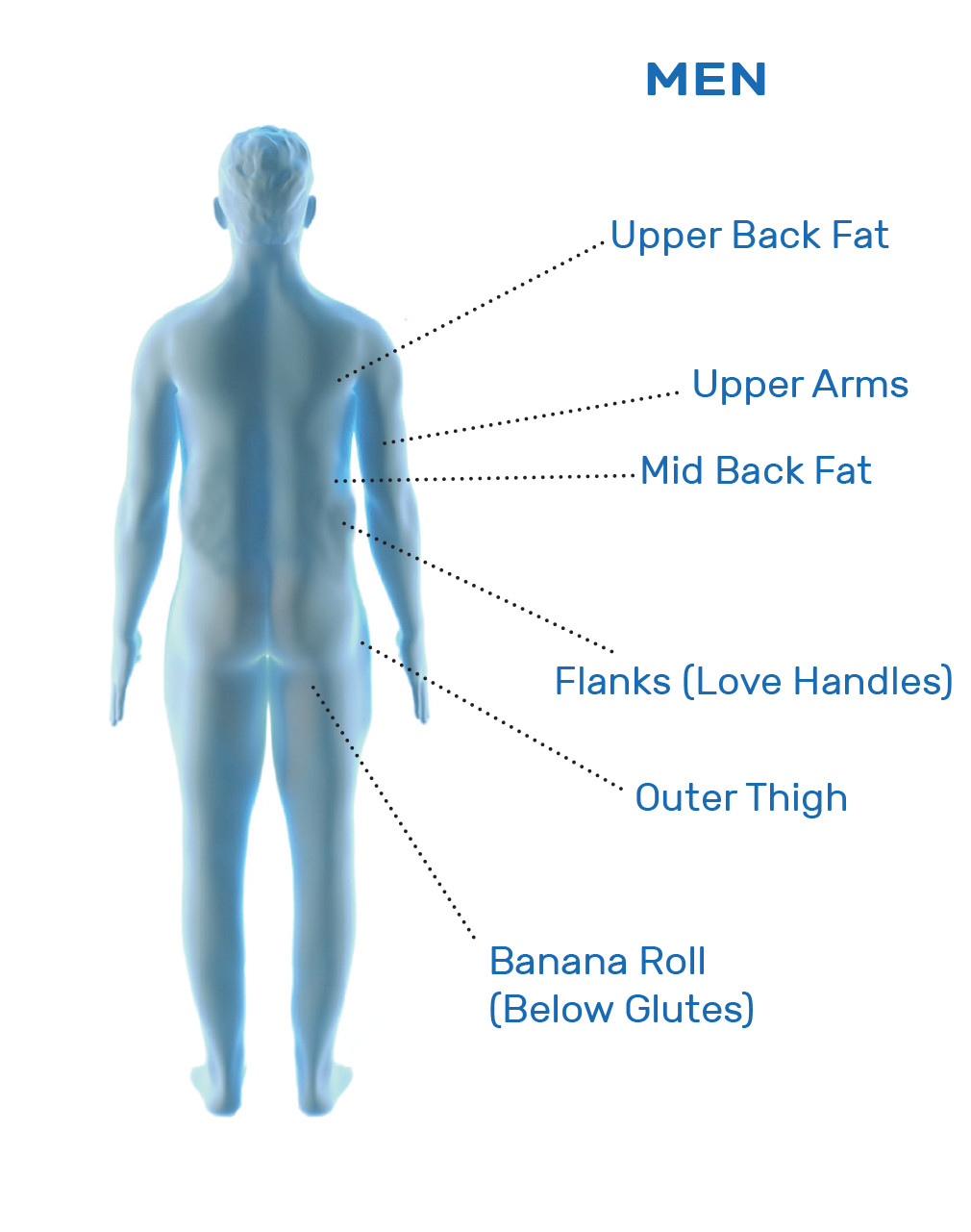
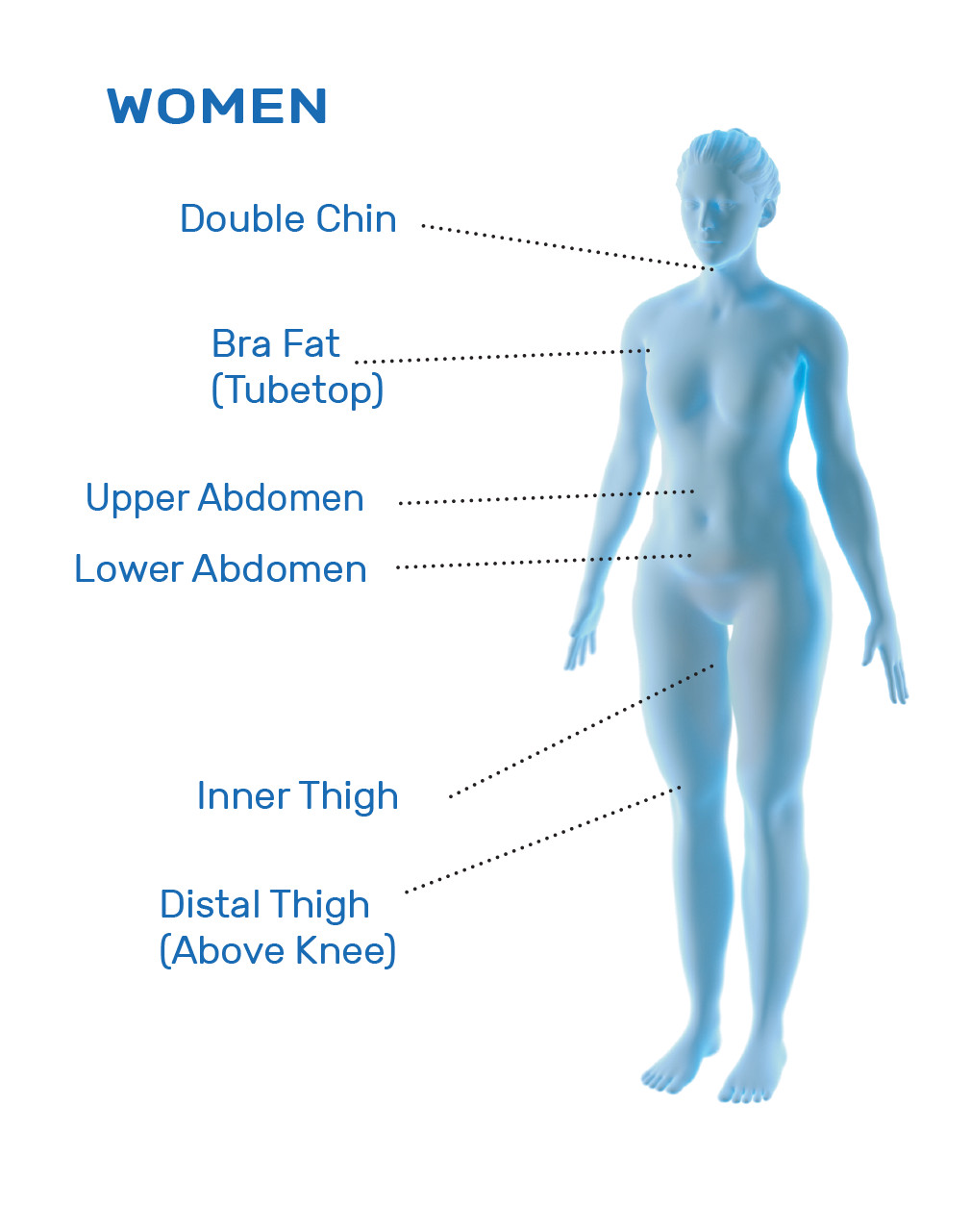
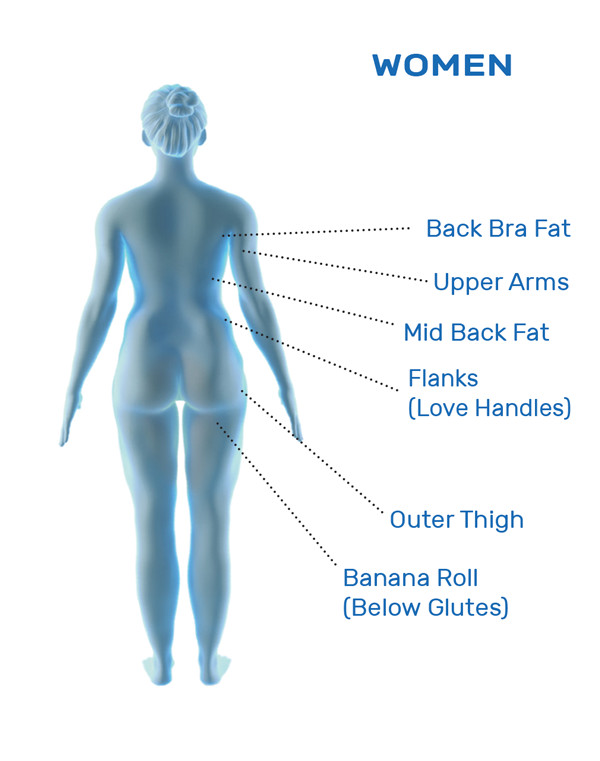
CoolSculpting® is FDA-cleared for the treatment of visible fat bulges in the submental (under the chin) and submandibular (under the jawline) areas, thigh, abdomen, and flank, along with bra fat, back fat, underneath the buttocks (also known as banana roll), and upper arm. It is also FDA-cleared to affect the appearance of lax tissue with submental area treatments. CoolSculpting® is not treatment for weight loss.
CoolSculpting® Important Safety Information
These procedures are not for everyone. You should not be treated with CoolSculpting® if you suffer from cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria.
Tell your doctor if you have any medical conditions including recent surgery, pre-existing hernia, and any known sensitivities or allergies.
During the procedure you may experience sensations of pulling, tugging, mild pinching, intense cold, tingling, stinging, aching, and cramping at the treatment site. These sensations subside as the area becomes numb. Following the procedure, typical side effects include temporary redness, swelling, blanching, bruising, firmness, tingling, stinging, tenderness, cramping, aching, itching, or skin sensitivity, and sensation of fullness in the back of the throat after submental or submandibular area treatment.
Rare side effects may also occur. CoolSculpting® may cause a visible enlargement in the treated area, which may develop 2 to 5 months after treatment and requires surgical intervention for correction.
Please see CoolSculpting® full Important Safety Information.
Patient Results May Vary.
Please view this link: Important Safety Information
“EMBODYMENT is the absolute best. They make this process more than comfortable, and always had my best interest in mind. They took their time in making sure that every single step was perfect, and have a great eye for beauty and aesthetic. I am so happy with my experience here, it’s changed my life. After my first consultation I left feeling valued and beautiful, but even more so after my treatment. I recommend Embodyment Coolsculpting® to any and everyone!”

LeighAnn is AMAZING! I did my research, watched all the videos available and came in with realistic expectations ... they were SURPASSED! I love my results and I could see and feel them within a couple of weeks. This is what I was hoping for and it made me feel so much better about myself. LeighAnn is kind and knowledgeable and honest about everything. On my last visit there was no need for her to try and sell me on something else - I was the one who asked about an additional procedure, I WANTED to give her more of my business. CoolSculpting was perfect for me because of some issues/hassle I have with blood-thinners - this was the perfect solution! I bruised (as expected), but I didn't have to worry about incisions or altering medications or recovery time, etc. I was back at work and feeling fine the next day! I'm doing a second area and I can't wait for my clothes to fit even better!

“I cannot recommend EMBODYMENT enough! From my initial consultation through the completion of my procedure, they prioritized my comfort. Weight loss can be a heavily sensitive subject, but EMBODYMENT had a way of making me feel beautiful in my skin, while validating my feelings for what I want out of CoolSculpting® to help me look and feel my best. They were detail-oriented and took the time to ensure that everything was done right to optimize my results. They are clearly masters in their craft, with extensive knowledge and experience. I trusted EMBODYMENT completely throughout the process and would recommended their skill to anyone considering CoolSculpting®!”

“I’m blown away by the incredible results that I have personally experienced with the Master Coolsculpting® specialist at EMBODYMENT. I’ve never had any type of cosmetic procedure before and was nervous to to do a treatment. I chose EMBODYMENT because I felt comfortable and confident with their extensive credentials and experience with Coolsculpting®. Now that I have treated my abdomen and “love-handles” and have already seen incredible results (dropped a full pant size), I’m a customer and believer for life. I can’t wait to treat other problem areas.”

“I had a wonderful experience at EMBODYMENT. To be honest I went to other places to check out the process, and I walked away from three of them completely convinced I would not trust them with my health and well-being. When I arrived they were courteous, kind, and took the time to answer all of my questions. They are extremely professional and willing to work with me through the fears I developed from the other consultations. In reality, there was no reason for the fears because they came from misinformation. The process was actually straightforward, and expedient. They always went above and beyond to ensure my comfort while treating me, of course the results exceeded my expectations. I looked forward to working with EMBODYMENT, and the choice was absolutely superior to the fancy multiple-staffed high end facilities that claimed to be “spas” but were evasive or uninformed about the process. They are experts you can trust, and they have the highest standards for your safety and comfort.”

“Great results! As soon as I turned 50 I started seeing areas of stubborn fat on my body that would not go away. I’ve never had that problem before so I was very discouraged. EMBODYMEN treated the areas around my torso that were particularly troubling. Now, three months later, the fatty areas that looked lumpy and took away from my hourglass figure are back to my pre-50’s normal. Would definitely recommend.”

Incredible experience! Embodyment exceeded my expectations, offering exceptional care and delivering outstanding results.
Consistent excellence and dedication. Embodyment sets the standard for quality and personalized service.
Dependable and professional. I wholeheartedly endorse their top-tier services for all clients.